Establishment License Application
Establishment License Application
What is an Establishment?
An establishment can either be a manufacturer, an Authorized Representative, AR (of a Foreign Manufacturer), an importer or a distributor of medical device. The complete definition of term “establishment” is given in Section 2 of Act 737. OEM or tendering agent is not defined as establishment.
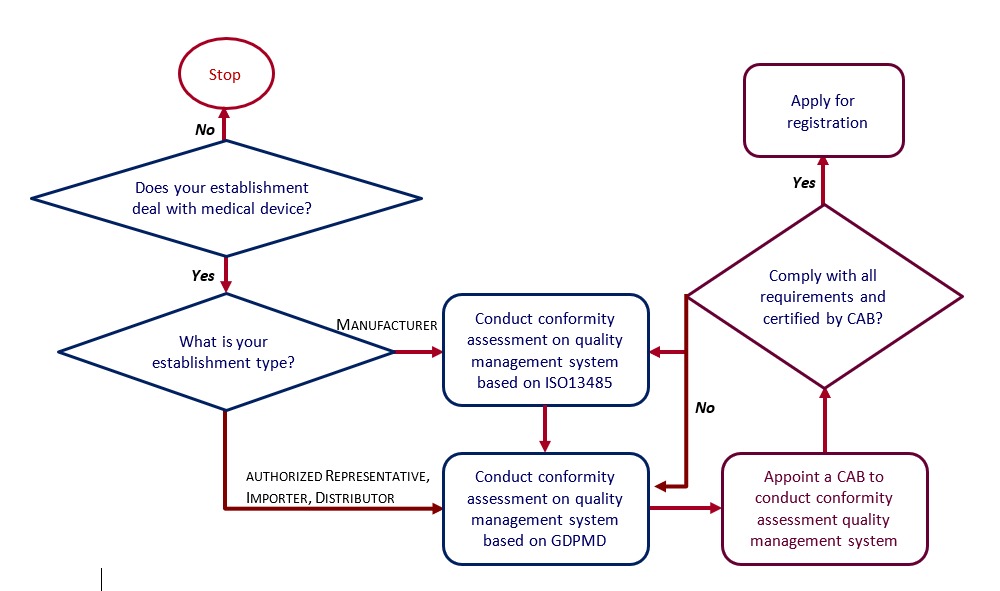
Figure 1 shows flow-chart of the steps to be taken by an applicant before making an application for an establishment licence under Act 737.
We have a team of consultants who are well-versed in the requirements and the MedCast System to assist you with your applications. The step-by-step guidance and consistent follow-up will help to expedite the application and approval process.
For Medical Devices MDQMS ISO13485:2016 & GDPMD License
How Long Is The Licence Validity And When We Can Start To Renew?
The validity of the establishment licence is 3 years and an establishment can start to renew the licence 1 year prior to expiry date.
Are We Allowed To Run Multiple Activities Under A Single Licence?
Based on the Circular No. 1 Year 2014, an establishment as the manufacturer of a medical device may carry out the activities of distributing manufactured medical device under a single licence.
Establishment that serves as the Authorised Representative may distribute and import medical device represented to them under a single licence.
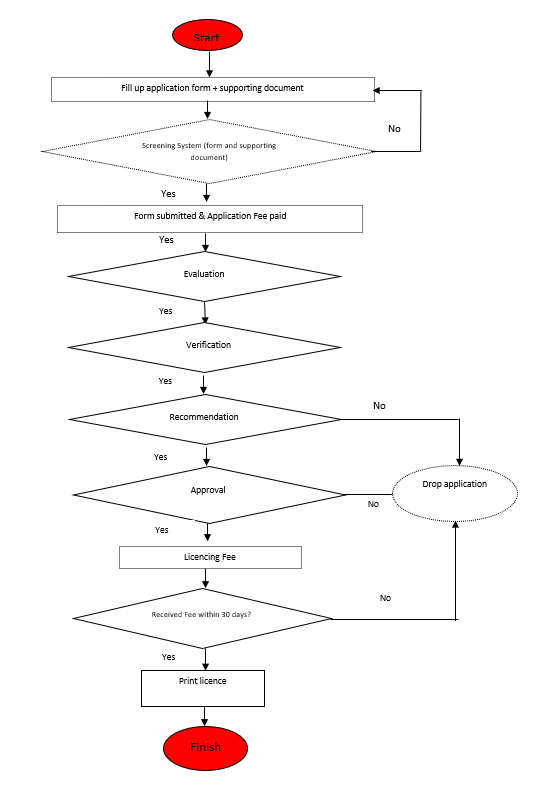
Figure 2 shows Process Flow for Submitting Establishment Licence Application:-
